Second Law of Thermodynamics
Second Law of Thermodynamics: Overview
This topic covers concepts, such as, Second Law of Thermodynamics, Terms in Second Law of Thermodynamics, Kelvin-Planck Statement & Clausius Statement etc.
Important Questions on Second Law of Thermodynamics
PMM2 is the machine which violates-
For an increase in the entropy define ____ of nature.
Entropy is the third law of thermodynamics.
Which of the following option are correct for the second law of thermodynamics?
What is the hypothetical example of the Kelvin – Planck Statement?
Kelvin Planck Statement deals with conversion of work into _____, conservation of work, conservation of heat, and conversion of heat into work.
Efficiency of a engine always less than unity.
An ideal gas heat engine operates in Carnot cycle between and . It absorbs cal of heat at high temperature. Amount of heat converted to work is:
Heat cannot by itself flow from a body at lower temperature to a body at higher temperature” is a statement or consequence of
State Clausius statement of second law of thermodynamics.
State Kelvin-Planck statement of the second law of thermodynamics
Write the statement of second law of thermodynamics
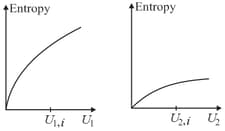
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
The change in entropy when a ice cube at is transformed into water at in is
Take ,
Clausius's statement is a part of
What do you mean by Clausius's statement?
The wavelength associated with the electron will be
